Shai Pilosof and his colleagues have bridged the disciplines of 'immunogenetics' and 'network ecology' for the first time. The study just published in Nature Communications demonstrates that the evolution of genes involved in immune response to parasites in one species depends on the whole web of host-parasite interactions in the system. Pilosof is a Ph.D. student in the Marco and Louise Mitrani Department of Desert Ecology at BGU's Jacob Blaustein Institutes for Desert Research.
In the course of evolution, hosts evolve immune response to parasites through genetic change while parasites evolve to overcome host defenses. In particular, genes of the Major Histocompatibility Complex (MHC), which exist in all vertebrates, including humans, are extremely important for an effective immune response because they encode for proteins that recognize foreign parasites such as viruses, bacteria or parasitic worms. One goal of ‘immunogenetics’, which is the field that explores the relationship between the immune system and genetics, is to understand the evolution of MHC under parasite-mediated selection.
Until now, the relationship between MHC evolution and parasitism has been explored mainly in systems of one parasite species in populations of a single host species. In nature, however, a particular parasite can infect many host species and a host species can be co-infected with many parasite species. This complexity in interactions is crucial because it creates indirect pathways by which infection of one host with a parasite depends on the infection status of other hosts. The best way to depict such complexity is by drawing a network of interactions (see figure). Such networks are extensively studied in the field of ‘network ecology’, which aims to understand the ecological and evolutionary processes underlying the structure of networks of species. Importantly, it is unclear how network structure affects MHC diversity, which in turn is one of the main drivers of host-parasites interactions.
In the recent paper, Pilosof and his colleagues explored this question using data on a community of 14 rodent hosts, their parasitic worms and their alleles of a MHC gene (allele is a molecular variants of a gene), collected in Southeast Asia. A comparison between the structure of a host-parasite network and that of a host-MHC allele network revealed that the structures are highly associated such that hosts infected with similar parasitic worms also harbored similar MHC alleles. A deeper analysis also discovered groups of MHC alleles and parasites that are more strongly linked to each other (within a group) than with alleles/parasites from other groups in the system. The structure of these groups depended on the structure of the host-parasite network, suggesting MHC-parasite co-evolution at the host community level, rather than at the level of a single species.
The study by Shai Pilosof and his colleagues shows that indirect effects between hosts and parasites affect MHC genetic diversity in more than one species, thereby scaling-up MHC theory from the population level to the community level. The study also has some applied implications because human-driven environmental changes can alter the interactions between hosts and parasites. For example, rodents and their associated parasites can invade disturbed areas, possibly creating new interactions between local rodents and invading parasites. Such perturbations to the host–parasite network may have consequences for the evolution of MHC through indirect cascading effects. Because rodents are carriers of zoonotic parasites (transferred from animals to humans), changes to network structure may have implications for risk of zoonotic diseases. This work opens new directions for future studies which can test such hypotheses.
Pilosof has recently been awarded the prestigious James S. McDonnell Foundation Postdoctoral Fellowship for his study of complex systems. The fellowship is awarded to only 10 PhD students worldwide, and Pilosof is the only Israeli to have received it this year. During his postdoctoral work, he will pursue research on "Diffusion dynamics in networks of networks". This field is at the forefront of science and embraces fascinating questions with important implications such as the spread of disease across different transport and social networks, the ecological relationships among different food webs and the relationships between different technological networks. The fellowship will allow him to advance this research frontier in Israel.
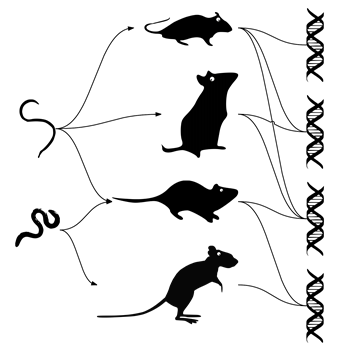
Above: An example for a host-parasite network (left) and a host-allele network (right). Arrows depict infection of a given host with a given parasite. Lines depict alleles harbored by given hosts.