The role of α-synuclein in synaptic transmission
α-synuclein is a small protein (14 KD) which is involved in Parkinson's Disease and other neurodegenerative conditions. It is an abundant protein which is highly enriched in the presynaptic terminal, where it associates with synaptic vesicles. It has been suggested to participate in SNARE-complex stabilization, synaptic vesicle clustering, endocytosis, vesicle recycling etc. Nevertheless, both its synaptic role and the manner in which it promotes neurodegeneration remain unclear. α-synuclein is prone to misfold, fomring fibrils and aggregates; the hallmark of Parkinson's Disease are Lewy bodies, large protein aggregates observed in the cell bodies of surviving dopaminergic neurons in the Substantia Nigra.
We are interested in the interaction between α-synuclein and another abundant group of presynaptic proteins, the synapsins. The synapsins are a family of proteins that associate transiently with synaptic vesicles and which play a major role in clustering them near the presynaptic active zone. In collaboration with Subhojit Roy, UCSD, we found that the well-documented inhibitory effect of α-synuclein on synaptic vesicle recycling is dependent on the presence of the synapsins and that the two proteins interact in-vitro. Interestingly, the Milovanovic group (Berlin) recently showed that α-synuclein is incorporated into synapsin droplets formed by liquid-liquid phase separation (LLPS).
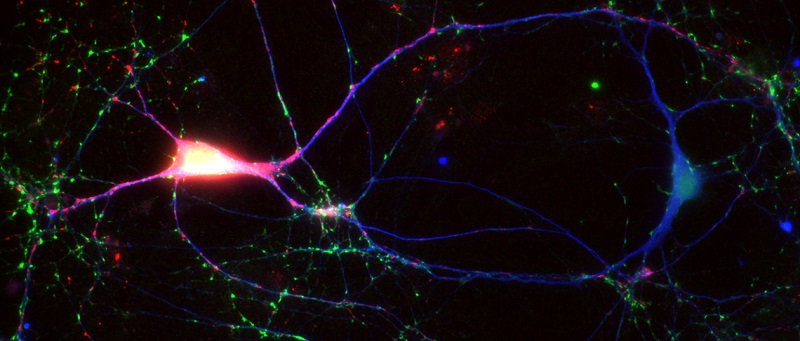
We are interested in the following topics concerning the function of alpha-synuclein in neurons:
1. With which of the various synapsin isoforms does α-synuclein interact? Are there functional consequences to differences in synapsin/α-synuclein interactions?
2. What is the interaction site between α-synuclein and synapsin?
3. Can we interfere with the interaction between these proteins? Can such interference reduce the synaptic effect of α-synuclein?
4. Can we identify variants or mutants of α-synuclein which function differently in synapses? Do they differ in their interactions with synapsin?
Different aspects of this research topic are supported by grants from the Israel Science Foundation (ISF), the Israel-USA Binational Science Foundation (BSF) and the National Institutes of Health of the USA (NIH), the latter two with Subhojit Roy.
Does the interaction between α-synuclein and the synapsins play a role in α-synuclein related pathogenesis?

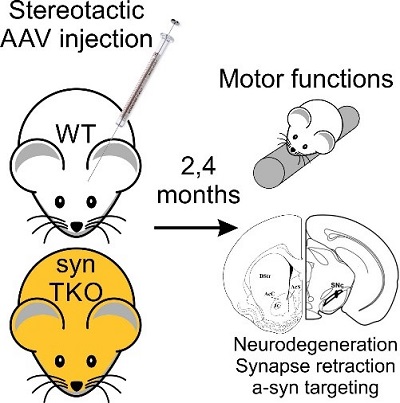
Virally-induced (AAV) overexpression of human α-synuclein in the substantia nigra in the midbrain of mice induces degeneration of dopaminergic neurons and produces deficits in motor function, mimicking some aspects of Parkinson's Disease. Our finding that the inhibitory effect of α-synuclein of synaptic transmission depends on the synapsins led to the question whether synapsin triple knockout mice, which lack all synapsins, may be resistant to the toxic effects of α-synuclein. We plan to determine whether this is the case by examining the effect of AAV overexpression of human α-synuclein in the brains of synapsin triple knockout mice and wild-type mice, followed by comparison of the survival of dopaminergic neurons in the substantia nigra and the development of motor deficits in the mice (with Claude Brosdki, BGU). On this basis, we further ask whether the synaptic effects of α-synuclein are related to its toxicity in the mid-brain.
This project is supported by a research grant provided by office of the vice-president for research of Ben-Gurion University (PIs: Daniel Gitler and Claude Brodski).
Anti-synapsin auto-antibodies in traumatic brain injury patients
In collaboration with Dr. Isaac Lazar (head of the pediatric intensive care unit of Soroka medical center) and Dr. Tehila Kaisman-Elbaz (Department of Neurosurgery, Soroka), we are currently investigating the prevalence of anti-synapsin autoantibodies in the serum of traumatic brain injury (TBI) patients. After informed consent, blood is collected from TBI patient who are admitted to Soroka hospital after accidents (at various time points) and are examined for the presence/development of anti-synapsin autoantibodies. Serum from control individuals is examined in parallel. Preliminary results show that a significant part of the TBI patients develop such antibodies, and that their prevalence in control individuals is substantially lower. We will continue examining this question, after which we will examine retrospectively if the presence of auto-antibodies correlates with long-term clinical outcomes. This research is based on our previous observation in collaboration with Dr. Markus Hötlje in Berlin, in which we found synapsin auto-antibodies in patients suffering from a wide-range of psychiatric and neurological disorders.
This project is supported by a grant from the Israel Ministry of Health (PIs: Daniel Gitler, Isaac Lazar and Tehila Kaisman-Elbaz).
Metabolic derailment of corneal sensory neurons in diabetes

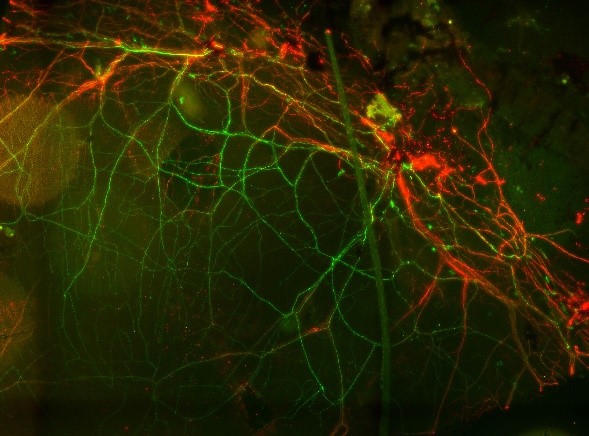 With Paul Fernyhough (St. Boniface Hospital, Winnipeg) we are examining the derailment of ATP production in sensory neurons of diabetic mice and rats. Using transgenic mice which express the genetically encoded ATP indicator ATeam1.03YEMK in neurons, including the sensory neurons of the cornea, we are measuring ATP in corneal sensory neurons in-vivo and asking if it is affected by experimentally-induced diabetes. The Fluorescence Resonance Energy Transfer (FRET) signal of ATeam is measured using a spectral confocal microscope in corneal sensory neurons. We are also determining the progression of the degeneration of the sensory neurons. Performed in collaboration with the department of Ophthalmology in Barzilai Hospital, Ashqelon. The image shows ATeam1.03YEMK (green) in sensory neurons in the cornea, also stained for beta-tubulin (red).
With Paul Fernyhough (St. Boniface Hospital, Winnipeg) we are examining the derailment of ATP production in sensory neurons of diabetic mice and rats. Using transgenic mice which express the genetically encoded ATP indicator ATeam1.03YEMK in neurons, including the sensory neurons of the cornea, we are measuring ATP in corneal sensory neurons in-vivo and asking if it is affected by experimentally-induced diabetes. The Fluorescence Resonance Energy Transfer (FRET) signal of ATeam is measured using a spectral confocal microscope in corneal sensory neurons. We are also determining the progression of the degeneration of the sensory neurons. Performed in collaboration with the department of Ophthalmology in Barzilai Hospital, Ashqelon. The image shows ATeam1.03YEMK (green) in sensory neurons in the cornea, also stained for beta-tubulin (red).This project is supported by a BGU-St. Boniface collaborative grant program (PIs: Daniel Gitler and Paul Fernyhough).
Presynaptic role of the mitochondrial calcium/sodium exchanger NCLX

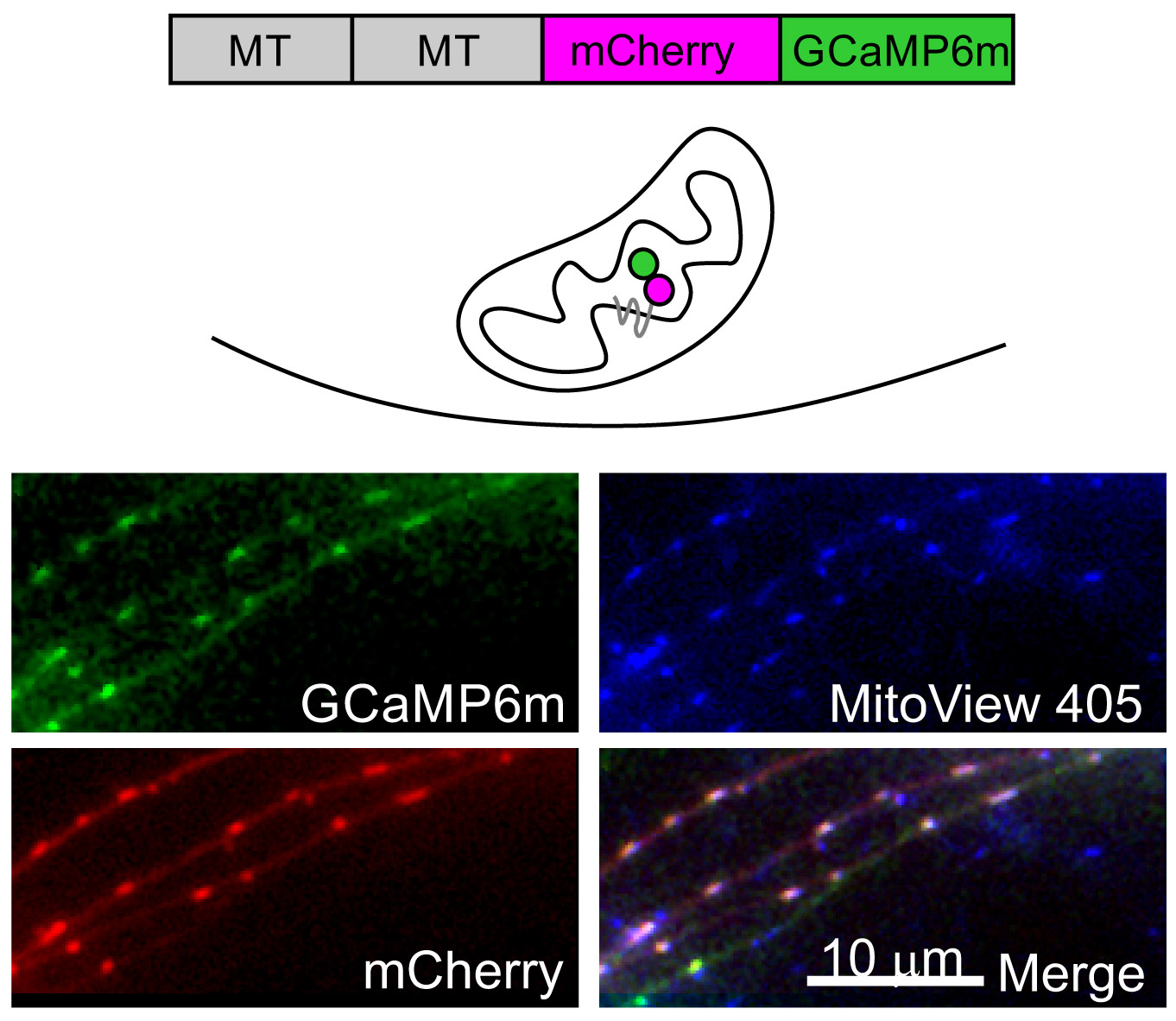 Calcium dynamics in the presynaptic terminal are key determinants of the properties of neurotransmission and of synaptic plasticity. In collaboration with Israel Sekler in our department, we are investigating how NCLX, the key extruder of calcium from the mitochondria, may control synaptic function. We investigated cytoplasmatic and mitochondrial calcium transients in NCLX knockout neurons and found that both neurotransmission, short-term plasticity and long-term potentiation are deleteriously affected by the deletion of NCLX. We are currently investigating the effect of NCLX and its P367S variant (which was found in human patients suffering from severe mental retardation) on neuronal development and synaptic structure and function.
Calcium dynamics in the presynaptic terminal are key determinants of the properties of neurotransmission and of synaptic plasticity. In collaboration with Israel Sekler in our department, we are investigating how NCLX, the key extruder of calcium from the mitochondria, may control synaptic function. We investigated cytoplasmatic and mitochondrial calcium transients in NCLX knockout neurons and found that both neurotransmission, short-term plasticity and long-term potentiation are deleteriously affected by the deletion of NCLX. We are currently investigating the effect of NCLX and its P367S variant (which was found in human patients suffering from severe mental retardation) on neuronal development and synaptic structure and function.