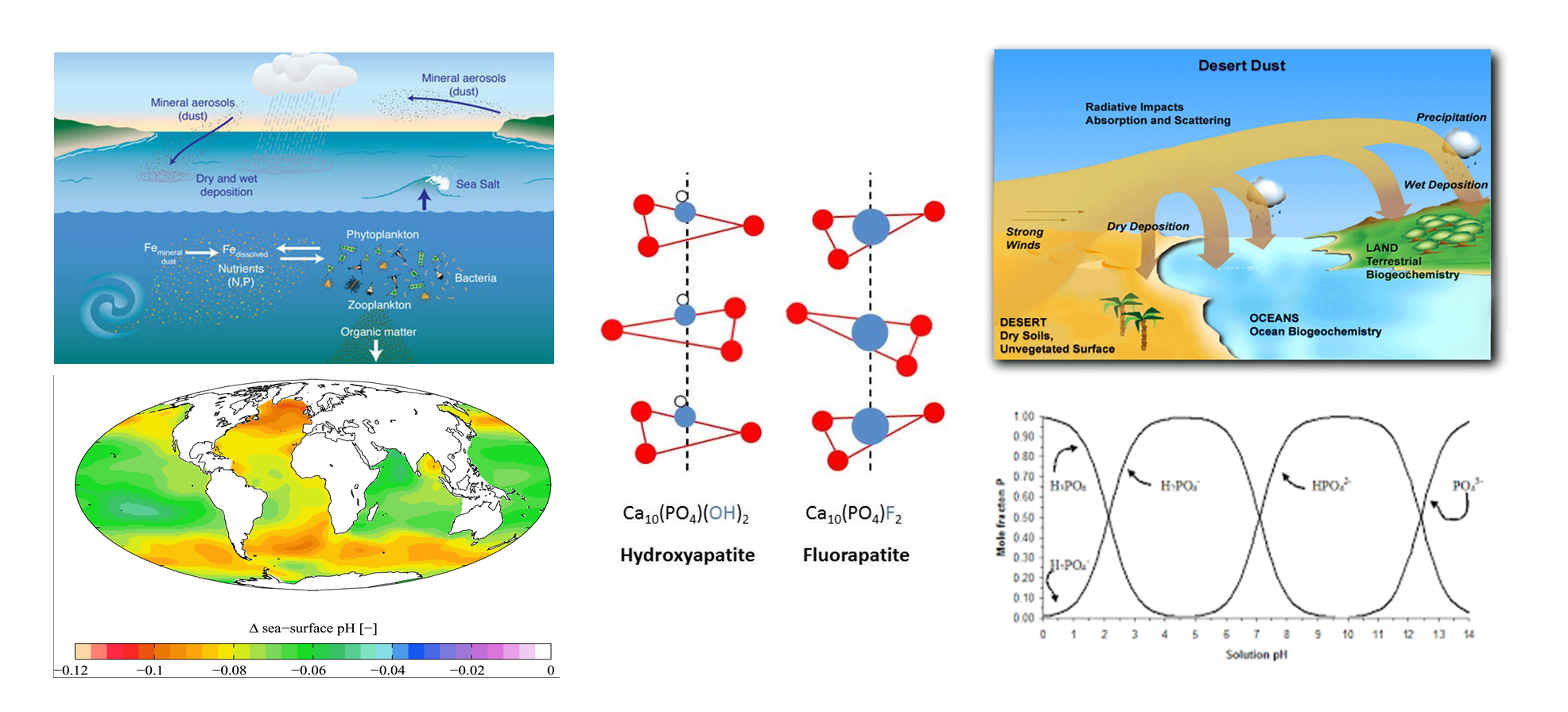
Ocean acidification and phosphorus solubility
The rise in atmospheric CO2 concentrations drive the decrease in Ocean pH levels, so-called 'Ocean Acidification'. Ocean acidification affect the cycling of major nutrients such as phosphorus (P). Unlike N, P and Fe does not have a gaseous phase and most of the new P and Fe that enters the oceans are transported from the continents with episodic desert dust storms. It has been proposed that dust is the most important P supplier in oceanic regions such as the Red Sea, the Mediterranean, and the Eastern and Central Atlantic Ocean, which are located downwind to major dust sources. Because these environments are limited or co-limited by P, dust is considered a major controller of the primary productivity in these ecosystems.
The bulk of the P in desert dust is in an inorganic mineral form and most of the P is usually bound to calcium (Ca) or Fe oxides and is poorly soluble under the moderately basic pH conditions of seawater, making much of the dust-P unavailable for planktonic organisms. The release of Ca or Fe bound P is controlled by abiotic processes and generally, dust-P solubility increases significantly as pH drops. Therefore, ocean acidification is expected to increase dust-P solubility.
In our lab we study how ocean acidification will change dust-P dynamics in seawater.